Why are there blue leaves: different light pigments for photosynthesis

By Leo Yuan Y10
Introduction to Photosynthesis
Plants use light energy to make two molecules needed for the next stage of photosynthesis: the energy storage molecule ATP and the reduced electron carrier NADPH.
The light reaction takes place in the thylakoid membranes of organelles called chloroplasts.
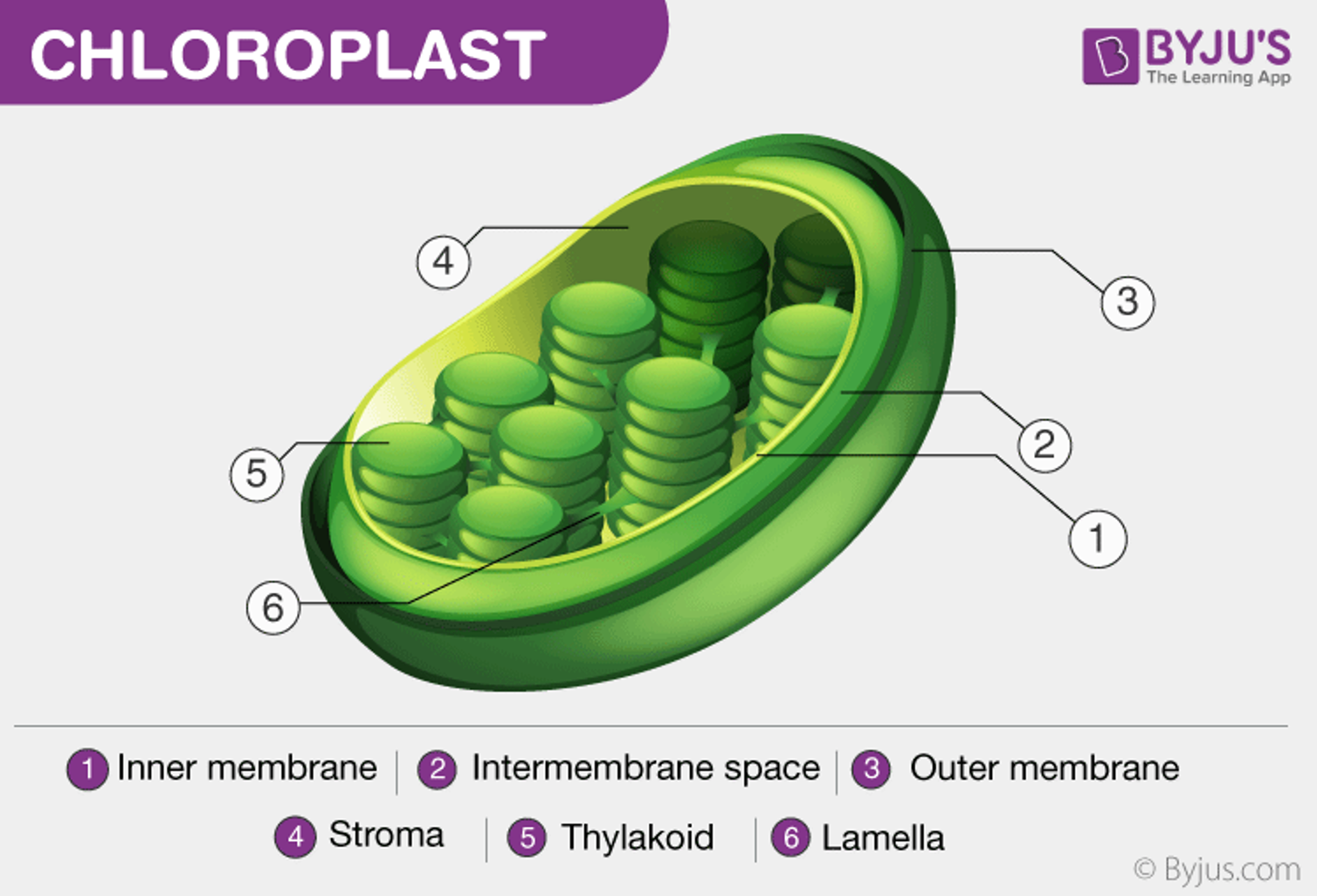
Photosystems: Large complexes of proteins and pigments (light-absorbing molecules) optimized to harvest light.
There are two types of photosystems: photosystem I (PSI) and photosystem II (PSII).
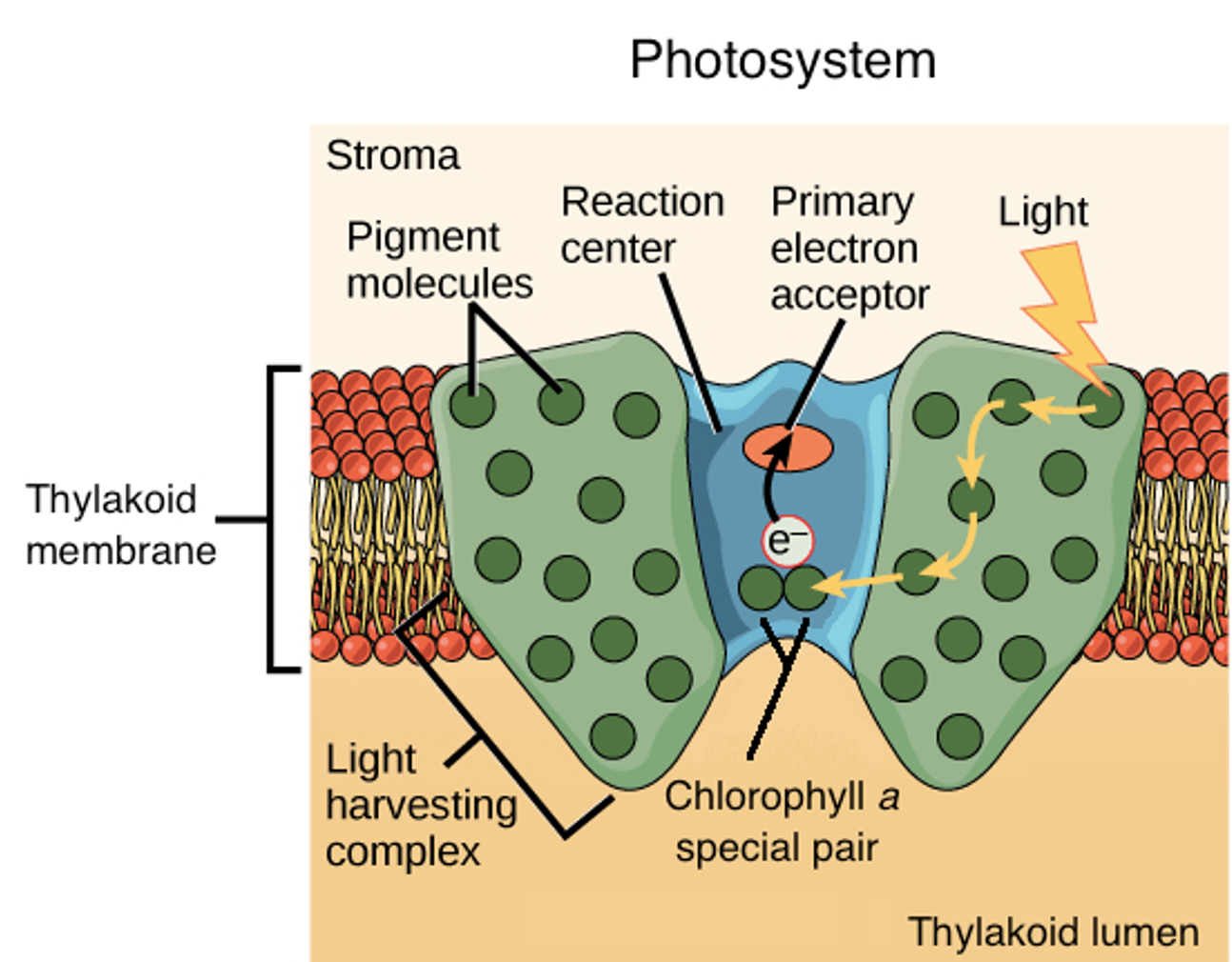
Characteristics of the photosystems:
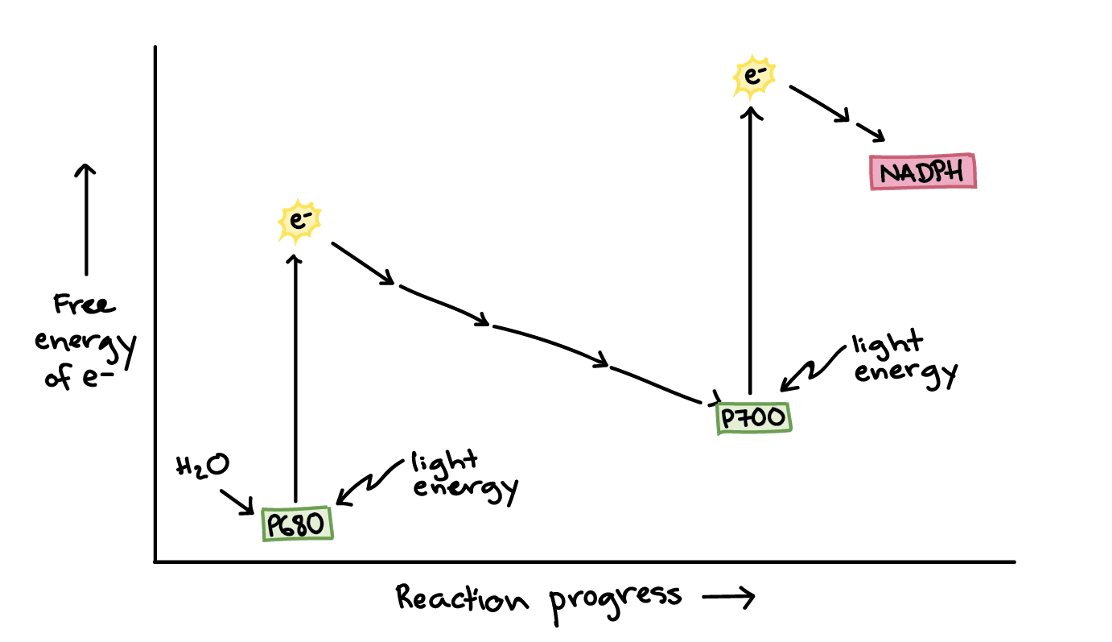
1. The PSII special pair absorbs best at 680 nm, while the PSI special absorbs best at 700 nm. Because of this, the special pairs are called P680 and P700,
2. Electron source: The PSII reaction center gets electrons from water, while the PSI reaction center is replenished by electrons that flow down an electron transport chain from PSII.
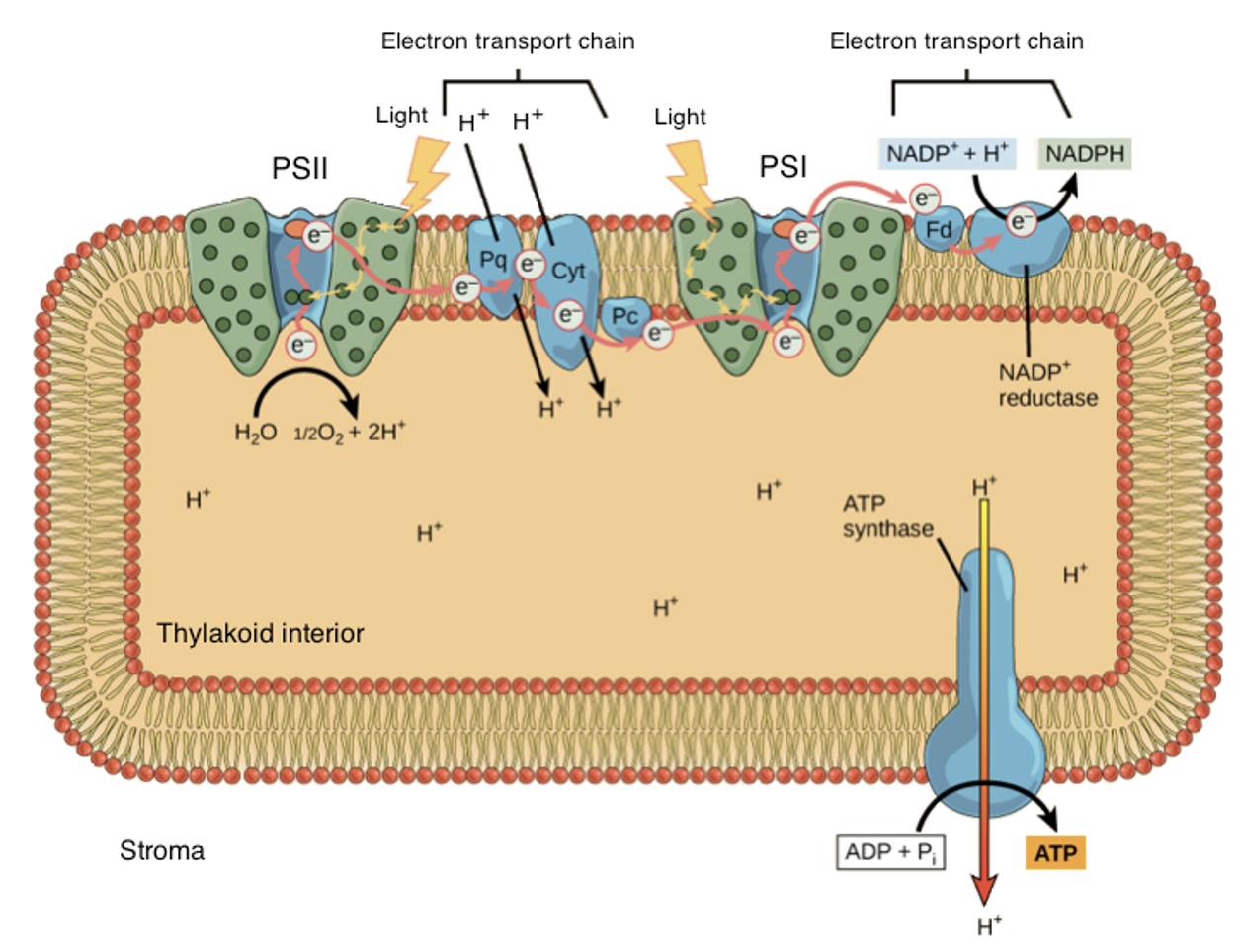
Process of light reaction:
1. A pigment absorbs a photon
2. Pigments raised to an excited state ( one of its electrons is boosted to a higher-energy orbital)
3. In a process called resonance energy transfer, Pigments transfer energy to a neighboring pigment through direct electromagnetic interactions.
4. The pigment molecules collect energy and transfer it toward a central part of the photosystem called the reaction center.
5. The reaction center has a unique pair of chlorophyll a molecules, often called special pair
6. The special pair can lose an electron when excited, passing it to another molecule in the complex called the primary electron acceptor.
7. The high-energy electron that passed to the acceptor molecule was replaced with an electron from water. This splitting of water releases the oxygen we breathe.
8. The high-energy electron travels down an electron transport chain, losing energy. Transport chains consist of a small organic molecule (plastoquinone, Pq), then a cytochrome complex (Cyt), and finally, a copper-containing protein called plastocyanin (Pc).)
9. The released energy drives the pumping of H+ ions from the stroma into the thylakoid interior, building a gradient.
10. H+ ions flow down their gradient and into the stroma, they pass through ATP synthase, driving ATP production in a process known as chemiosmosis.
11. Electrons get to photosystem 1
12. Light triggers photosystem 1 to work and the electron in P700 is boosted to a very high energy level and transferred to an acceptor molecule.
13. The high-energy electron travels down a short second leg of the electron transport chain. The electron is first passed to a protein called ferredoxin (Fd), then transferred to an enzyme called NADP+reductase. At the end of the chain, the electron is passed to NADP+ to make NADPH.
14. NADPH will travel to the Calvin cycle, where its electrons are used to build sugars from carbon dioxide.
Different pigments in plants:
The absorption spectra of three key pigments in photosynthesis: chlorophyll a, chlorophyll b, and β-carotene:
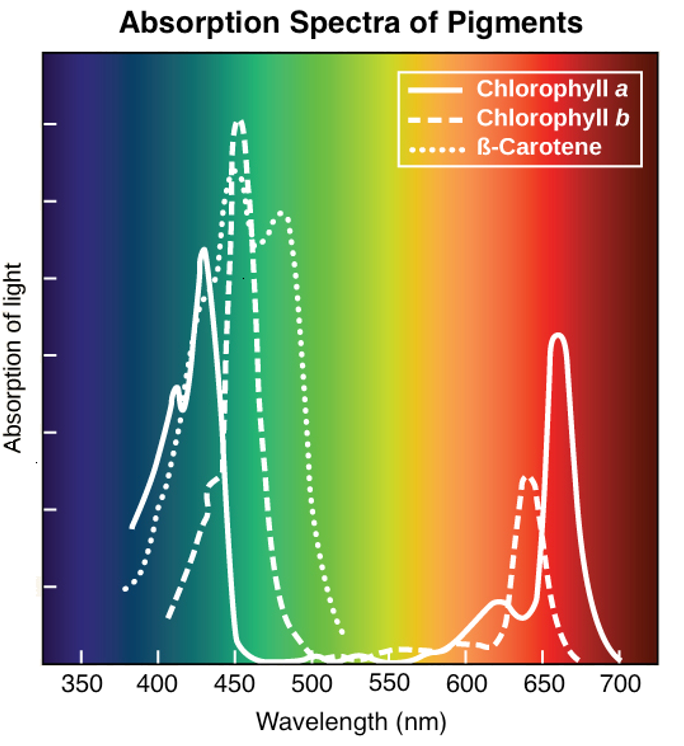
The set of wavelengths that a pigment doesn't absorb is reflected, and the reflected light is what we see as color.
Eg. plants appear green to us because they contain many chlorophyll a and b molecules, reflecting green light.
Two types of pigments:
Chlorophyll:
Five main types of chlorophylls: chlorophylls a, b, c, and d, plus a related molecule found in prokaryotes called bacteriochlorophyll.
Chlorophyll molecules absorb blue and red wavelengths.
Structurally, chlorophyll molecules include a hydrophobic tail that inserts into the thylakoid membrane and a porphyrin ring head (a circular group of atoms surrounding a magnesium ion) that absorbs light.
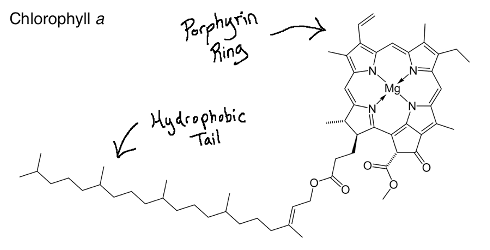
All photosynthetic plants, algae, and cyanobacteria contain chlorophyll a, whereas only plants and green algae contain chlorophyll b, along with a few types of cyanobacteria.
Accessory pigments allow a broader range of wavelengths to be absorbed, and thus, more energy to be captured from sunlight.
Carotenoids:
Functions:
1. absorb violet and blue-green light
- often used to attract animals, which can help disperse the plant's seeds.
3. Carotenoids in chloroplasts help absorb the excess energy and dissipate it as heat.
Only a photon with an appropriate energy to bump an electron between orbitals can excite a pigment. So pigments absorb specific light that has a specific wavelength.
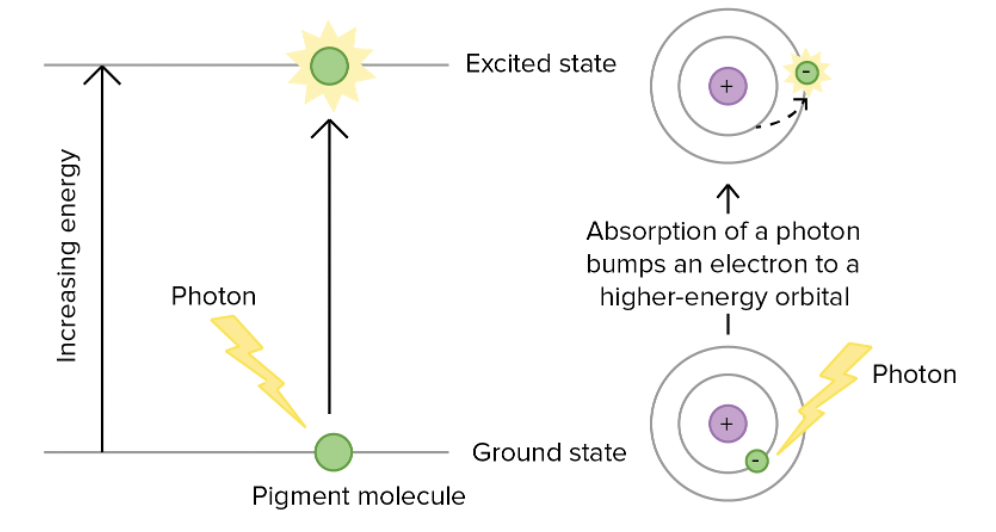
The "energy gaps" between the orbitals are different in each pigment, meaning that photons of different wavelengths are required to provide an energy boost that matches the gap.